

A facile and economical strategy is designed to enhance the adsorption affinity of kaolin toward phosphate by acquiring calcium (Ca) and magnesium (Mg) from seawater. The modified kaolin is prepared at different temperatures to obtain CaO/MgO with different surface properties. The different modified kaolins are comparatively characterized using X‐ray diffraction, zero charge, BET surface area, energy dispersive spectrometry, and X-ray fluorescence spectroscopy. Solution pH slightly affected the removal of phosphate by modified kaolin in the range of 4-7. The maximum adsorption capacity of the modified kaolin sintered at 600 °C (KS600) was 4.07 mg g-1, which is eleven times higher than that of raw kaolin. The presence of SO42- or CO32- ions demonstrates a negative effect on adsorption, whereas the presence of Cl-, F-, or NO3- exerted a negligible effect on removal efficiency. Importantly, KS600 had the capacity to remove phosphate effectively in real sewage. The strong interaction between phosphate and CaO/MgO played a key role in the enhancement of phosphate adsorption. These results indicate that the abundant ocean resources could be used for environmental remediation.
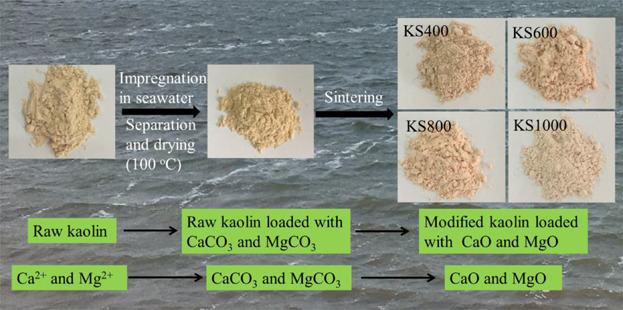
Scheme for the preparation of modified kaolin.