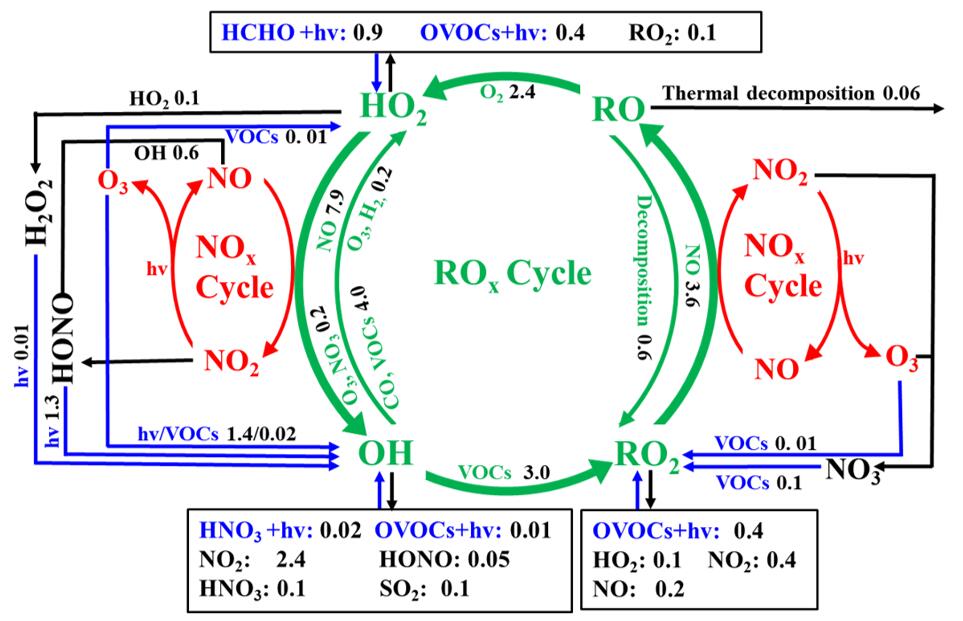
A typical multi-day ozone (O3) pollution event was chosen to explore the atmospheric oxidation capacity (AOC), OH reactivity, radical chemistry, and O3 pollution mechanism in a coastal city of southeastern China, with an observation-based model coupled to the Master Chemical Mechanism (OBM-MCM). The hydroxyl radical (OH) was the predominant oxidant (90±25%) for daytime AOC, while the NO3 radical played an important role in AOC during the nighttime (72±9). Oxygenated volatile organic compounds (OVOCs; 30±8%), NO2 (29±8%), and CO (25±5%) were the dominant contributors to OH reactivity, accelerating the production of O3 and recycling of ROx radicals (ROx=OH+HO2+RO2). Photolysis of nitrous acid (HONO, 33±14%), O3 (25±13%), formaldehyde (HCHO, 20±5%), and other OVOCs (17±2%) was a major ROx source, which played an initiation role in atmospheric oxidation processes. Combined with regional transport analysis, the reasons for this O3 episode were the accumulation of local photochemical production and regional transport. The results of sensitivity analysis showed that volatile organic compounds (VOCs) were the limiting factor of radical recycling and O3 formation, and the 5% reduction of O3 would be achieved by decreasing 20% anthropogenic VOCs. Controlling emissions of aromatics, alkenes, and alkanes with ≥4 carbons was beneficial for ozone pollution mitigation. The findings of this study provide significant guidance for emission reduction and regional collaboration for future photochemical pollution control in the relatively clean coastal cities of China and similar countries.